Oncology Practices
Improve Visit Adherence, Reduce Cost of Care, and Participate in the Enhancing Oncology Model.
With Value-Based Care models expanding, Guideway’s Service as a Solution addresses the clinical and non-clinical barriers that dramatically impact performance and patient satisfaction. Through payment models like EOM, providers are held accountable for care quality and experience, which are often predicated on addressing health related social needs to lower, practical, informational, emotional, physical, familial, and spiritual barriers to care.
Driving financial performance, while achieving health equity, through care guidance in Oncology.
Decrease in ICU Utilization – 3 Year Published Study
Details of the Enhancing Oncology Model
According to the CMS release, fact sheet, and RFA, the EOM is a 5-year voluntary model, beginning on July 1, 2023, that aims to improve quality and reduce costs through payment incentives and required participant redesign activities. CMS designed EOM to test how to improve health care providers’ ability to deliver care centered around patients, consider patients’ unique needs, and deliver cancer care in a way that will generate the best possible patient outcomes.
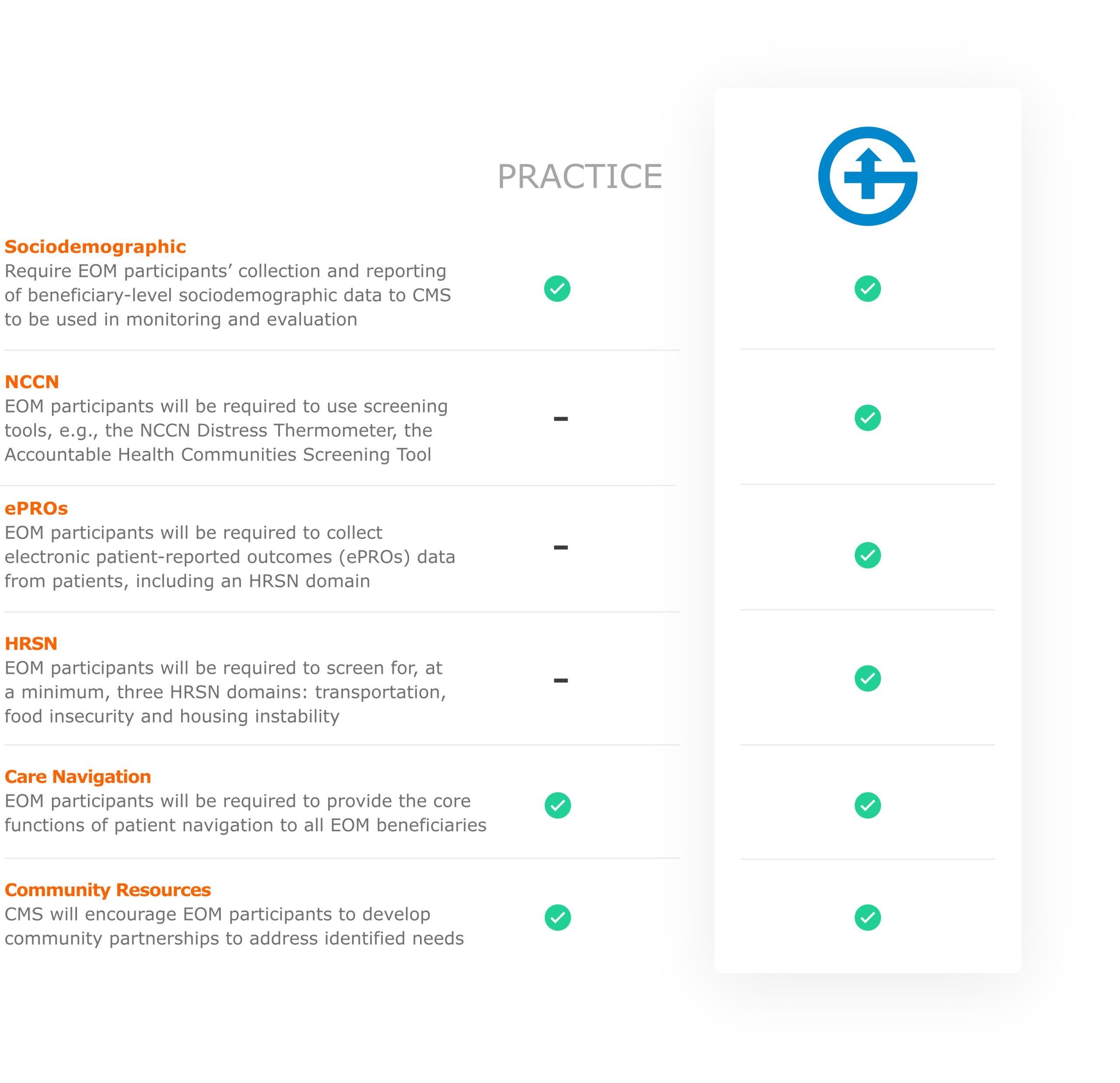
Under EOM, participants are incentivized to consider the whole patient and engage with them proactively, during and between appointments. EOM participants are required to implement participant redesign activities, including 24/7 access to care, patient navigation, care planning, use of evidence-based guidelines, use of electronic Patient Reported Outcomes (ePROs), screening for health-related social needs, use of data for quality improvement, and use of certified electronic health record technology. As part of the use of data for quality improvement, participants will submit health equity plans to CMS, where EOM participants will detail their evidence-based strategies to mitigate health disparities identified within their beneficiary populations.
Key Elements of the Model
-
Comprehensive, coordinated cancer care
-
Continuous improvement driven by data
-
Payment incentives including a Monthly Enhanced Oncology Services (MEOS) payment, and a performance-based payment (PBP) or a performance-based recoupment (PBR)
-
An aligned multi-payer structure
-
Focused efforts to identify and address health disparities.
Delivering a 3-8x ROI
for Cancer Programs
-
90th percentile patient satisfaction ratings
-
Significant reductions in staffing expense
-
Seamless data integration (EHR)
-
58% improvement in new patient visit adherence for Medicare patients
-
30% decrease in ED visits for cancer patients
-
55% decrease in hospitalizations for cancer patients
-
60% decrease in ICU admissions for cancer patients

How We Improve Patient Satisfaction, Decrease Operational Expense, and Lower Total Cost of Care
Guideway Care recently celebrated its 10th year partnering with health care organizations to solve health equity related patient barriers. The company’s origins began through a $15 million CMMI grant to prove the thesis that lay cancer navigators could improve quality, patient satisfaction and decrease cost. The program was highly successful (delivered $57 million in savings over three years across five states) and its outcomes were published in JAMA.
Since then, Guideway has continued its work in oncology, but has also evolved to serve most other areas of Value-Based Care (VBC).
For one oncology program operating under a VBC model for three years, Guideway Care Guides had 70K encounters with 6k patients, resolved 98% of 6K barriers, with an average time to resolution of 9.6 days, logged 135K activities, and drove greater than 50% improvement in initial visit compliance.
During that time Guideway captured 5.1M data points.
How We Achieves Results with Partner Organizations
Guideway Care hires and extensively trains Care Guides who successfully address SDoH and disparity-related barriers. Care Guides build peer-to-patient relationships that allow for the identification and resolution of non-clinical barriers and the escalation of clinical barriers to the proper clinical teams. This peer-to-patient relationship leads to a uniform care experience that creates value for all stakeholders in the care continuum.
Guideway studies have shown that more than 80% of the identified barriers do not require clinical escalation, which substantially improves capacity within the partner’s clinical team.
Promptly addressing the physical, practical, emotional, informational, spiritual and familial barriers that impact patients both improves clinical quality and decreases cost. The Care Guides’ use of Guideway’s proprietary platform, which guides and informs patient activation, is critical to not only the success of the peer-to-patient relationship, but also captures and communicates relevant data back to healthcare organizations (such as those data elements required under the EOM).
Not only is the platform critically important for capturing SDoH data and disparity related barrier resolution, but it is also very important for operational improvement. For example, Guideway can validate impact and performance.
Why Partner with Guideway Care in the Enhancing Oncology Model
The American Association for Cancer Research recommends an equitable path forward for all cancer patients, which entails the use of patient advocates and navigators who can resolve SDoH-related disparities, the vast majority of which are non-medical in nature. To further illustrate this point, the recently released CMS Enhancing Oncology Model RFA contains the following number of references: 48 to disparities, 40 to health equity, 31 to patient navigation, 18 to care management and 5 to SDoH. Section IV. Health Equity Strategy (page 13-17) within the RFA details the program goals and requirements.
Guideway Care’s seamless approach to EOM will ensure program goals are met, patient satisfaction is improved, operating expenses are lowered, and total cost of care metrics are met.
Enhancing Oncology Crosswalk to Health Equity
Key Benefits
-
Lower cost of delivery
-
Improved patient satisfaction
-
Data integration (EHR)
-
Improved patient capture, compliance, and persistence
-
Lower total cost of care (acute care avoidance)
-
10+ Years in oncology care navigation
Learn more about how we partner with oncology practices to drive performance.
Email us or contact us via LinkedIn.


